A liver-targeting pleiotropic drug candidate targeting multiple types and stages of liver disease
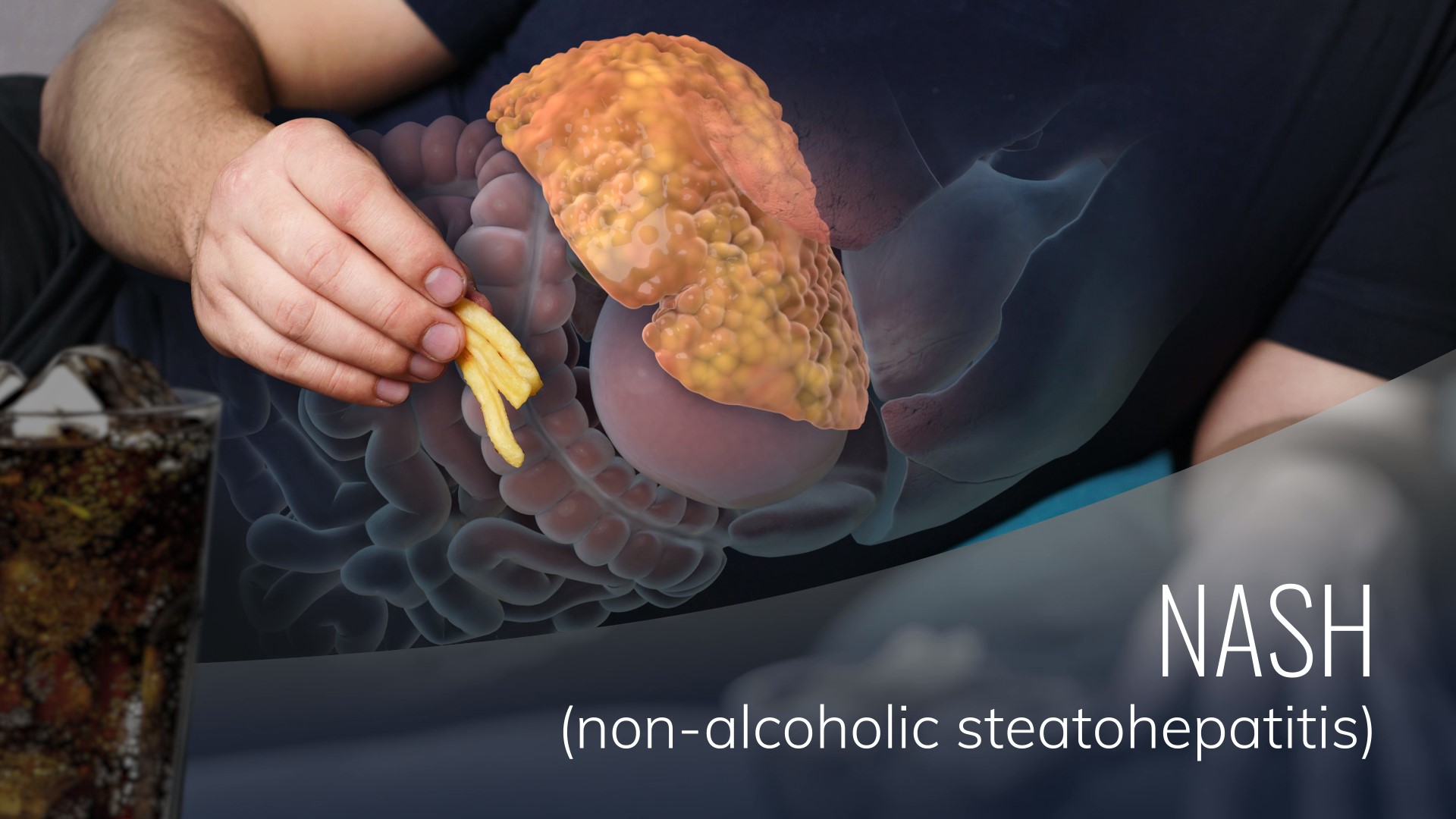
Rencofilstat is Hepion’s clinical phase, lead oral drug candidate for nonalcoholic steatohepatitis (NASH) and viral hepatitis-induced liver disease. Rencofilstat meets all of Hepion’s criteria for an effective and unique pleiotropic liver disease drug. As a novel molecule chemically derived from a pharmacological class of therapeutic drugs used clinically for over 35 years, Rencofilstat has thus far demonstrated an excellent safety profile in all animal and clinical studies to date. Thanks to multiple, diverse mechanisms of action and its accumulation in the liver (5-times higher concentrations than in the blood), Rencofilstat has demonstrated efficacy in two distinct animal models of NASH and HBV replication. These findings are consistent with in vitro studies documenting mechanisms of action as an antiviral agent (HBV, HCV, HDV ), protection from cellular stress and death, anti-steatotic, anti-inflammatory, anti-fibrotic, and anti-cancer activities. Thus, Rencofilstat is positioned to become a highly versatile therapeutic drug for the most prominent liver diseases of our time. No other marketed drugs or NASH drug candidates in development address all these mechanisms.

Rencofilstat
Candidate Rencofilstat Mechanisms of Action in Chronic Liver Disease (NASH and Viral Hepatitis)
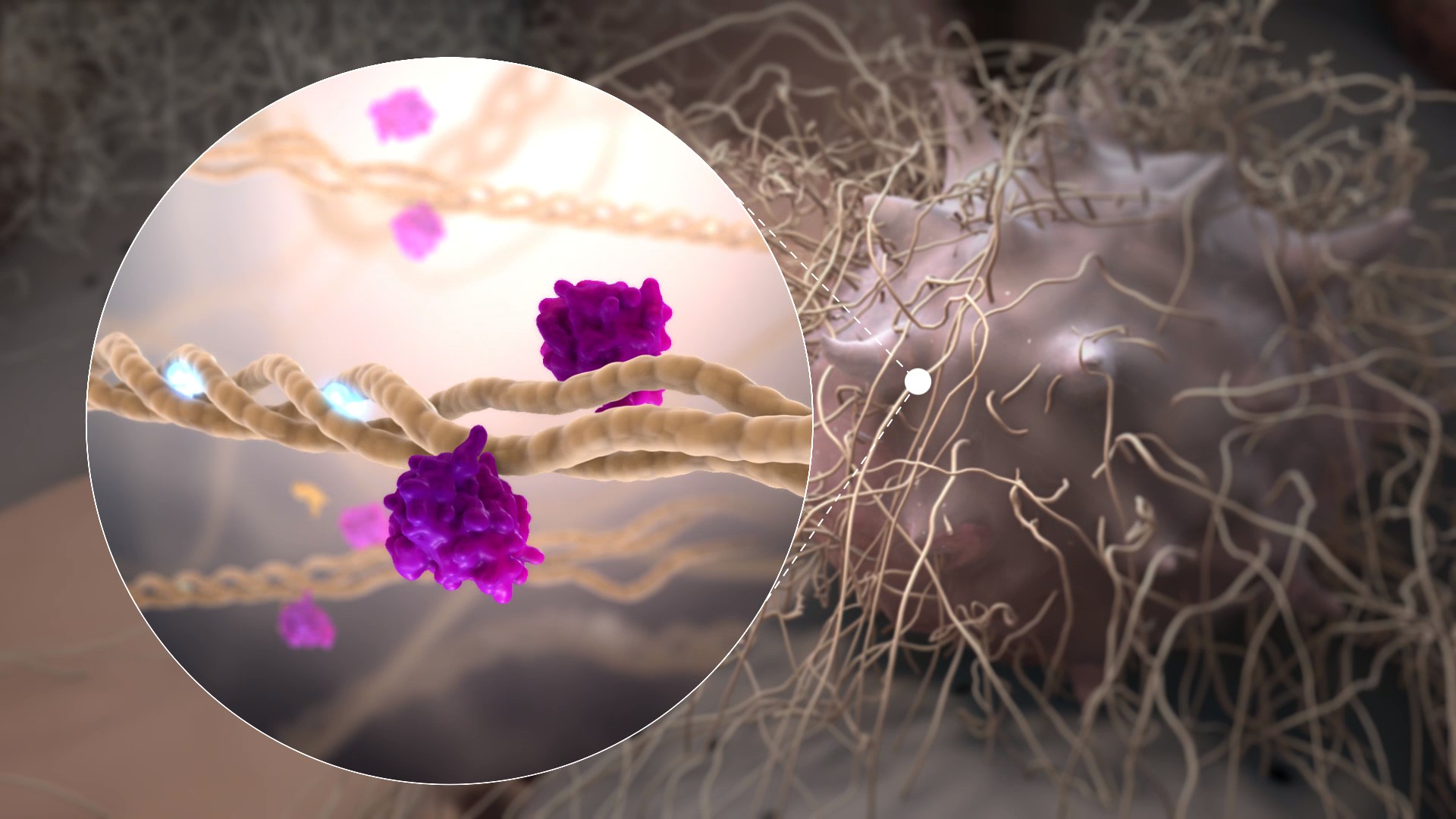
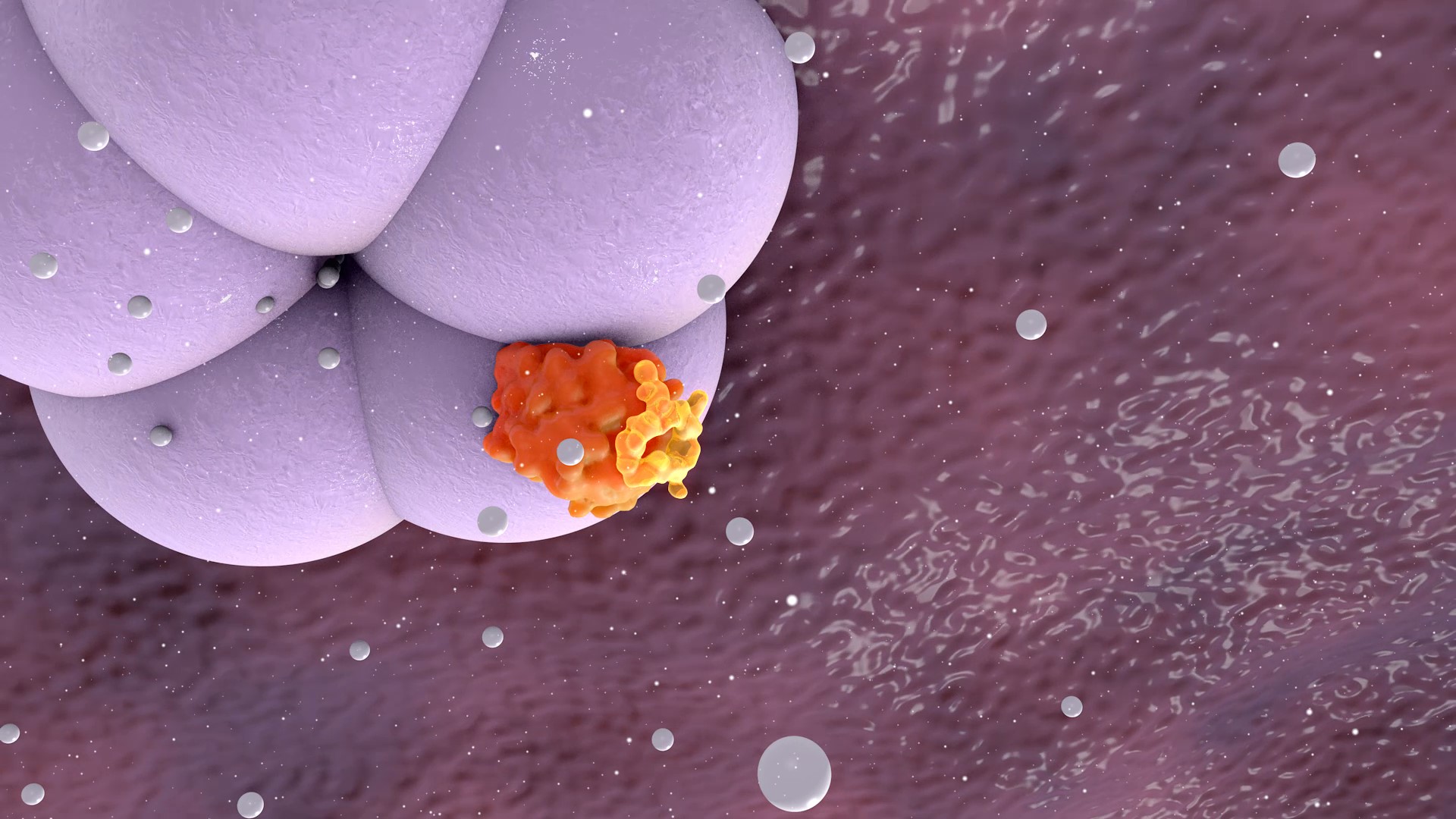
Rencofilstat specifically binds cyclophilin isomerase enzymes and inhibits cyclophilin function with the highest known potency of any reported cyclophilin inhibitor (Ki= 1 nM). The pleiotropic actions of Rencofilstat are due to the fact that multiple cyclophilin isoforms exist in the body and participate in many biological processes. Cyclophilins assume especially prominent roles in disease processes such as cell death, fibrosis, and cancer cell growth and metastasis. Many viruses have evolved to recruit cyclophilins into their life cycles to assist in viral replication and evade the immune system. By blocking the participation of cyclophilins in these processes, Rencofilstat displays a variety of therapeutic activities.
Rencofilstat Binds to Cylophilin A
Rencofilstat Blocks the Action of Cylophilin B
Rencofilstat Binds to Cylophilin D
Learn More
Cyclophilin Isoform |
Disease Stage |
Mechanism of Action |
| A | Viral Infection | Block virus entry, replication, and other activities that trigger liver injury |
| D | Cellular Injury | Decrease mitochondrial stress, ER stress, cell death |
| D | Steatosis | Decrease lipogenesis by lowering transcription of sterol regulatory element–binding protein-1c |
| A | Inflammation | Decrease infiltration and activation of inflammatory cells by blocking extracellular cyclophilin A-CD147 interaction |
| B | Fibrosis | Decrease collagen synthesis and pro-fibrotic activation of hepatic stellate cells |
| B | Fibrosis | Decrease collagen hydroxylation and crosslinking |
| A | Cancer | Decrease cancer cell adaptations to hypoxia |
| A | Cancer | Suppress metastasis-related gene expression |
| A | Cancer | Suppress signaling pathways regulating cancer cell proliferation |
Rencofilstat consistently decreased NASH-associated fibrosis in several studies, and multiple cyclophilin-mediated mechanisms may have contributed to the effect. Anti-fibrotic activity is an important advantage of Rencofilstat because many NASH drugs in development instead primarily target mechanisms regulating steatosis during the early phase of NASH. Fibrosis often is well established by the time that patients are diagnosed with NASH, and furthermore, fibrosis is the strongest predictor of adverse clinical outcomes in fatty liver disease, including liver-related death. Consequently, the FDA in a recently amended guidance for drug developers has highlighted noncirrhotic NASH with liver fibrosis as the area of greatest need and potential effect on health (Noncirrhotic Nonalcoholic Steatohepatitis With Liver Fibrosis: Developing Drugs for Treatment, Guidance for Industry, December 2018).
Inflammation, fibrosis, and carcinogenesis are as prevalent in viral hepatitis-induced liver disease as they are in NASH. The additional capacity of Rencofilstat to inhibit the life cycle of multiple hepatitis viruses means that Rencofilstat can simultaneously target the driving force of virus-induced liver disease as well the pathological activities that manifest in damaged livers. Antiviral activities of Rencofilstat apply to hepatitis B, C, and D viruses as well as other, non-liver-tropic viruses such as human immunodeficiency virus-1, and these activities occur through a variety of mechanisms (Gallay et al., 2015, PLoS One 10(8):e0134707). For example, Rencofilstat decreases HBV levels by blocking virus uptake into cells as well as blocking post-entry stages of the replication cycle. Experimental data has thus indicated reductions in HBV DNA, HBsAg, HBeAg, and pgRNA. In the case of HCV infection, Rencofilstat blocks the interaction between cyclophilin and the viral protein, NS5A, which is important for HCV replication.